FOR IMMEDIATE RELEASE
Media Contact:
Joana Casas, Program Communications Manager
(212) 806-1602 (212) 806-1602; joana.casas@amfar.org
(212) 806-1602; joana.casas@amfar.org
amfAR Report Examines Controversial Pricing of Hepatitis C Treatment
Calls for better balance between cost of drug development, profit margins, and public health needs

Download the full report here.
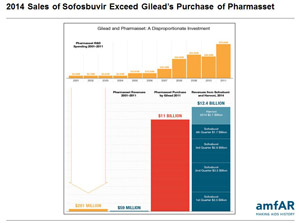
Download PowerPoint Slides NEW YORK, Feb. 17, 2015 – Despite important price reductions for some low- and middle-income countries, the exorbitant drug pricing of breakthrough treatments for hepatitis C (HCV) infection will inevitably limit access to the drugs, leading to unnecessary loss of life, according to a new report from amfAR, The Foundation for AIDS Research. While the new drugs have made curing HCV easier and more effective, the report finds that the aggressive pricing of the treatments will place an unjustifiable and unsustainable burden on healthcare systems in the U.S. and around the world, with potentially devastating consequences for people living with HCV.
Globally, as many as 185 million people are living with hepatitis C, including more than three million Americans. HCV is a blood-borne disease that can be transmitted sexually and perinatally as well as through needle sharing, unsterilized medical equipment, and contaminated blood products. Chronic HCV infection can lead to serious liver problems, including cirrhosis (scarring of the liver) or liver cancer. It is estimated that up to 350,000 people die every year due to HCV-related liver disease.
The new amfAR report, titled “Hepatitis C and Drug Pricing: The Need for a Better Balance,” urges structural changes that alter the pricing incentives for pharmaceutical companies in such a way that they cannot charge exorbitant prices for their products, however effective they may be. Among the HCV drugs highlighted in the report is sofosbuvir, which is priced at $1,000 a pill, or $84,000 for a 12-week course of treatment in the U.S. Marketed as Sovaldi by the pharmaceutical company Gilead Sciences, sofosbuvir has a much higher cure rate, is easier to administer, and has fewer side effects than older hepatitis C treatments.
“Due to their astronomical cost, it is unfortunate that effective treatments for hepatitis C will remain largely out of reach and unaffordable for most people with hepatitis C, especially among populations in resource-limited settings,” said Kevin Robert Frost, amfAR Chief Executive Officer. “We cannot let the pursuit of huge profits undermine access to these life-saving treatments for those who need them. The healthcare system in the U.S. is broken, and the unregulated pricing of drugs is a significant factor contributing to its demise. If we are serious about making health care more affordable for everyone in the U.S. and around the world, a change must be made now, or else the magnitude of this problem will only worsen.”
The controversial pricing of the HCV medications has prompted much criticism among patient advocacy groups, healthcare providers and government officials, including a bipartisan group of U.S. Senators, who have requested evidence to support the company’s pricing as part of an investigation into Gilead. The company earned $12.4 billion in 2014 alone from sales of sofosbuvir and Harvoni (ledipasvir/sofosbuvir).
The report also points to the unsustainable burden of HCV drug pricing on U.S. and global healthcare systems. In the U.S., hepatitis C patients are mostly uninsured, underinsured and/or incarcerated. Even with some form of health insurance coverage, the new HCV drugs will still be out of reach to many because Medicaid, Medicare, the Department of Veterans Affairs and the prison system are limiting access to them because of their high cost. It is estimated that treating all HCV-infected individuals in the U.S., even with all available discounts, would cost approximately $110 billion.
The report finds that even wealthy countries, including Canada, the United Kingdom and Germany, are finding the price of sofosbuvir difficult to bear. Gilead recently licensed seven pharmaceutical companies in India to manufacture generic formulations of sofosbuvir in 91 developing countries. But for the millions of people in the 51 middle-income countries not covered by the licenses, the treatment will likely remain all but inaccessible.
“The advent of these drugs that can cure hepatitis C is a groundbreaking achievement, but the irrational pricing makes it virtually impossible to treat millions of people around the world who are disproportionately affected, in particular, the five million living with HIV who are co-infected with hepatitis C,” said Greg Millett, amfAR Vice President and director of public policy. “Innovative strategies and open dialogue between pharmaceutical companies, national governments, patient advocates and other stakeholders will be needed to solve the problem of how to deliver effective and affordable hepatitis C treatment to the people who need it most.”
The full report, Hepatitis C and Drug Pricing: The Need for a Better Balance, is available on amfAR’s web site at www.amfar.org.
About amfAR
amfAR, The Foundation for AIDS Research, is one of the world’s leading nonprofit organizations dedicated to the support of AIDS research, HIV prevention, treatment education, and the advocacy of sound AIDS-related public policy. Since 1985, amfAR has invested nearly $400 million in its programs and has awarded grants to more than 3,300 research teams worldwide. For more information, please visit www.amfar.org.