CTLs: Stormtroopers of the Immune System
By Marcella Flores, MPH, Ph.D., Associate Director of Research, amfAR
 Dr. Jones is the recipient of the first amfAR grant to be funded by generationCURE, a group of young professionals dedicated to advancing amfAR’s efforts to find a cure for HIV.A major obstacle to HIV eradication is the presence of a reservoir of virus that is established soon after infection and lies dormant during effective antiretroviral therapy. Eradicating the cells that harbor that virus is the goal of research aimed at a sterilizing cure (in which all virus is completely eliminated from the body). One approach being taken by researchers to accomplish such a cure is to recruit the help of a subset of immune cells charged with clearing the reservoir.
Dr. Jones is the recipient of the first amfAR grant to be funded by generationCURE, a group of young professionals dedicated to advancing amfAR’s efforts to find a cure for HIV.A major obstacle to HIV eradication is the presence of a reservoir of virus that is established soon after infection and lies dormant during effective antiretroviral therapy. Eradicating the cells that harbor that virus is the goal of research aimed at a sterilizing cure (in which all virus is completely eliminated from the body). One approach being taken by researchers to accomplish such a cure is to recruit the help of a subset of immune cells charged with clearing the reservoir.
Natural killer (NK) cells and cytotoxic T cells (CTLs) mediate the killing of virally infected cells and, in the case of CTLs, can be specific for HIV. Much research has been devoted to retraining CTLs to be better killers of HIV-infected cells. Writing in the February issue of The Journal of Clinical Investigation, Drs. Brad Jones and Bruce Walker review the properties of CTLs that could be exploited as part of a cure for HIV.
Dr. Jones is Assistant Professor in the Department of Microbiology, Immunology and Tropical Medicine at The George Washington University. He gained expertise in CTLs during his postdoctoral work in the lab of Bruce Walker, who was one of the first to describe CTLs in HIV-infected persons. Dr. Jones has published numerous studies on CTLs and continues to pursue his research with a grant from amfAR’s generationCURE. Here, he shares his views on the latest approaches being used by scientist to harness CTLs in the search for a cure for HIV.
You recently published a review article with Dr. Bruce Walker on the role of CTLs in HIV cure studies. What makes this topic especially timely?
CTLs, also known as CD8+ T cells, are cells of the immune system that specialize in recognizing and killing virus-infected cells. I always find it inspiring to see these guys in action—moving quickly through tissues and hunting for virus-infected cells. When a CTL encounters an infected cell it rapidly discharges toxic proteins, killing the target cell, essentially eliminating a virus factory. The sensitivity and specificity of this killing is really impressive.
Even more impressive is the impact CTLs can have in a clinical setting. Louis Picker, Jonah Sacha, and colleagues in Oregon, for example, have developed a means of eliciting a CTL response that is capable of completely eradicating SIV (the monkey version of HIV) from animals. The CTLs elicited in this study have a very unusual way of recognizing infected cells, but serve to illustrate the power of these types of cells if they can be properly directed.
“I always find it inspiring to see these guys in action—moving quickly through tissues and hunting for virus-infected cells.”
The challenges involved in either completely eradicating HIV infection (sterilizing cure) or enabling the immune system to control infection long-term (functional cure) are both considerable. In my opinion, our best chance at achieving these goals centers on finding ways to aim and enhance the natural antiviral weapons in our immune system, such as CTLs, against persistent virus.
Why do CTLs fail to clear HIV infection?
There are several factors at play here. The classical explanation would be that CTLs cannot recognize the viral reservoir because it hides in a latent state. This is certainly part of the answer, but I would speculate that there is some ongoing interaction between CTLs and the viral reservoir, even in individuals with undetectable viral loads.
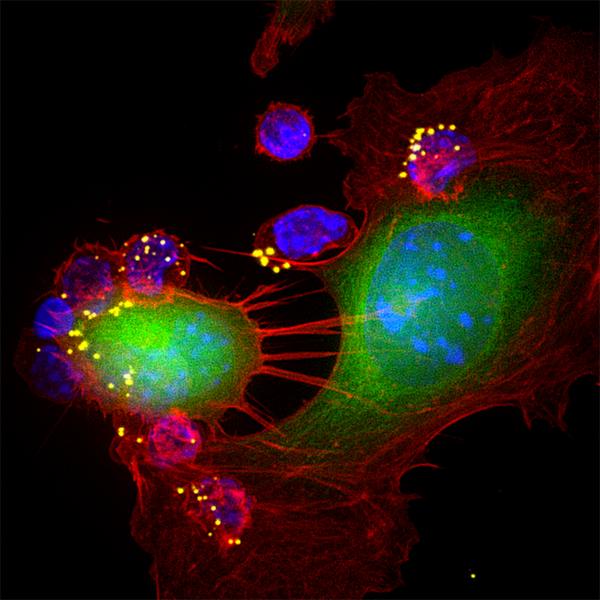
An award-winning image of CTLs attacking a tumor cell (Photo:Sudha Kumari)
Another part of the explanation is that HIV has an uncanny ability to mutate in order to ‘escape’ detection by CTLs. We also know that CTLs become ‘exhausted’ when faced with persistent infections such as HIV and less effective at eliminating infected cells. However, there is still a lot that we don’t know. Several labs, including my own, are interested in determining what makes a CTL particularly effective against the viral reservoir so that we can hopefully elicit these responses therapeutically.
What are some of the approaches scientists are using to make CTLs more effective?
I would highlight two studies that I am involved in. In one project funded by amfAR’s generationCURE, we are studying a drug that has the potential to act as both an immunomodulation agent and a latency-reversing agent – thus simultaneously exposing infected cells to CTLs and enhancing the ability of CTLs to kill these targets. In a second project we are exploring immunomodulation using an antibody that enhances the activity of IL-21, a molecule that improves the killing ability of CTLs.
Definitions:
Immunomodulation: The use of drugs or biologics (antibodies, cytokines, etc.) to alter the immune response.
Latency reversal: Latency-reversing agents wake up dormant HIV and facilitate elimination of infected cells.
 Dr. Michel Nussenzweig is working with colleagues to test broadly neutralizing antibodies in a combination cure approach in an amfAR-supported clinical trial.Are there any CTL-focused trials currently in the clinic?
Dr. Michel Nussenzweig is working with colleagues to test broadly neutralizing antibodies in a combination cure approach in an amfAR-supported clinical trial.Are there any CTL-focused trials currently in the clinic?
Yes, there are quite a few trials of therapeutic HIV vaccines aimed at enhancing CTL responses, including some that combine vaccination with latency-reversing agents to try to ‘shock and kill’ the reservoir. Cath Bollard, in collaboration with David Margolis, is also in the process of testing CTL therapy – whereby HIV-targeting CTLs are isolated from participants, expanded and then re-infused, with the hope that these will drive reductions in the reservoir. I’m anxiously awaiting the results from these studies. If we can see a signal that the reservoir is significantly reduced in some individuals, then we will have something that we can grab onto and further improve upon – this is really the breakthrough that we are all working towards.
What strides have been made in HIV cure research that make you hopeful that we will find a cure, sterilizing or functional, in the near future?
In regard to a sterilizing cure, I would again point to the work of Louis Picker, Scott Hansen, Jonah Sacha and others at Oregon Health Sciences University with their demonstration that an unusual type of T cell response is able to eradicate SIV infection from monkeys. There are some key limitations of the current form of this intervention – first, the vaccine has thus far been given before an animal is infected and, second, it is only effective in approximately 50% of animals. However, to my knowledge, this is the first and only example of immune-mediated elimination of infection with a lentivirus.
“I commend amfAR for being highly involved in pushing this research forward.”
Stepping away from CTLs for a moment, I am also encouraged by the work being performed with broadly neutralizing antibodies as reported by Michel Nussenzweig, James Whitney, Dan Barouch, and others. We have an incredible arsenal of very effective antibodies available and evidence is mounting that these can be used to target the viral reservoir. I commend amfAR for being highly involved in pushing this research forward.
Gilead has presented exciting data showing that a small-molecule TLR-7 agonist is able both to reverse HIV latency and to enhance the functions of both CTLs and NK cells, representing a potentially powerful combination.
In relation to a functional cure, the phenomenon of post-treatment control of HIV for individuals who started treatment early in infection, best represented by the VISCONTI cohort in France, provides an important precedent that long-term immune control is possible. If the mechanisms underlying this control can be determined, then we will have a path forward to attempt to induce the appropriate responses in a broader array of individuals.
There is really a tremendous amount to be excited about in this rapidly moving field and we are extremely fortunate to be working with a community that is supportive of our efforts, both in terms of financial support and by volunteering to participate in clinical studies. As long as we continue to pull together, we will achieve the goal of curing HIV – either through one of the approaches highlighted above or, perhaps, through something entirely unexpected.