amfAR Fellows Make Important Leaps in Vaccine Research
March 2, 2005—HIV has baffled researchers for 20 years. Because of its remarkable ability to avoid destruction by the immune system, the virus has made the development of an effective AIDS vaccine a very elusive goal so far. But two amfAR research fellows have achieved separate breakthrough discoveries that promise to change the landscape of vaccine research and help bring us closer to an effective AIDS vaccine.
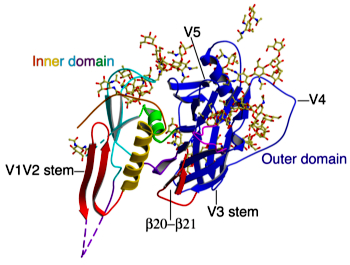
Dr. Chen's diagram detailing the complexity of the gp120 protein |
n the February 24 issue of the prestigious journal Nature, Dr. Bing Chen, working with colleagues at Harvard University, described for the first time the structure of the gp120 protein on the surface of the virus before it makes its first contact with target cells. The research paper details the massive shape changes the protein undergoes after it makes that initial cell contact.
“Knowing what gp120 looks like before it binds to human cells gives us some of our best clues as to what a vaccine should look like,” explained Dr. Chen. “HIV in its unbound state, before it attaches to CD4, is what we need to go after in terms of generating the most effective immune responses against the virus.”
Dr. Chen’s research was called “a technical tour de force” in an accompanying editorial in Nature by Dr. Peter Kwong, a prominent vaccine researcher at the National Institutes of Health’s Vaccine Research Center. Researchers have been trying to generate pictures of unbound gp120 for 20 years but, as with other aspects of the virus, it has proven to be a slippery target—one that Dr. Chen has finally managed to pin down. While HIV has many tricks up its sleeve, this work by Dr. Chen and his colleagues gives researchers a new tool in the search for a fatal weakness that could be exploited in the design of an AIDS vaccine.
Also in February, another amfAR research fellow, Dr. Rosa Cardoso, working with colleagues at the Scripps Research Institute in La Jolla, California, described the structure of a “broadly neutralizing” antibody, which was discovered in a patient roughly ten years ago and since that time has challenged researchers attempting to understand how it works. Neutralizing antibodies are proteins secreted by immune cells and are particularly important in eliminating many infections. This antibody, called 4E10, is described as broadly neutralizing because it can disable, or neutralize, a wide range of HIV strains. Most researchers feel that the ability of a vaccine to elicit antibodies will be the most important characteristic of an AIDS vaccine to prevent HIV infection.
Dr. Cardoso’s report, which appeared in the February issue of the journal Immunity, represents the first successful determination of the structure of this broadly neutralizing antibody and the first physical description of how it interacts with the virus. Why is this important? By knowing what the antibody looks like, and by extension knowing the structure of the region of HIV to which the antibody binds (known as the epitope), researchers could design mimics of the epitope, which can be used as the basis for an AIDS vaccine.

Dr. Bing Chen |
|
Both the Chen and Cardoso studies have received significant media attention, including coverage by Reuters, Scientific American, Nature News, and other news sources.